electron configuration of chromium|Electron Configuration For Cr : Cebu Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the . Enter your delivery Pincode. . Fish has come back on our table thanks to Jalongi. Trisha Dey. No Stinky Hands. Fresh fish. No hassle to visit the market. Save time. I am ordering from quite a long time now. No pain of cutting and washing. Recipe Ideas. Club. Useful Link. About Us; Why Jalongi; Blogs; Refer & Earn; Terms & Conditions .
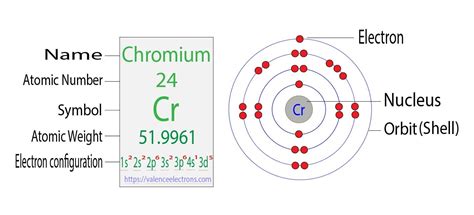
electron configuration of chromium,Learn how to write the electron configuration for chromium and its ions using the period table or an electron configuration chart. See the correct and incorrect ways to arrange the electrons in orbitals and the reason for the exception of chromium.
Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the .Key Takeaways: The electron configuration of chromium (Cr) is [Ar] 3d^5 4s^1. Chromium has a total of 24 electrons, with 5 electrons in the 3d orbital and 1 electron .Key Takeaways: Chromium’s electron configuration is [Ar]3d^5 4s^1. It contains a total of 24 electrons divided among different orbitals and subshells. The electron .
electron configuration of chromium Electron Configuration For Cr Learn why chromium has the electron configuration [Ar]3d54s1 and not [Ar]3d44s2. See explanations based on exchange energy, coulombic repulsion energy, and orbital size.Learn about the electron configuration of chromium, a lustrous, hard metal with a silver-grey color. Find out its chemical properties, uses, facts, and FAQs on this web page.For example, the observed ground state electron configuration of chromium is [Ar] 4s 1 3d 5 rather than the predicted [Ar] 4s 2 3d 4. Similarly, the observed electron configuration . Chromium electron configuration in an easy language is basically the process in which the chemical element distributes its electrons in a given system of .Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers .For example, the observed ground state electron configuration of chromium is [Ar]4s 1 3d 5 rather than the predicted [Ar]4s 2 3d 4. Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9.In several cases, the ground state electron configurations are different from those predicted using th periodic table and the Aufbau Principle. Some of these anomalies occur as the 3d orbitals are filled. For example, the .The electron configuration of chromium (Cr) is [Ar] 3d^5 4s^1. Chromium has a total of 24 electrons, with 5 electrons in the 3d orbital and 1 electron in the 4s orbital. Chromium is an exception to the normal electron configuration rules due to the stability of having a half-filled 3d orbital.

The answer is [Ar] 4s1 3d5Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled 4s and half-filled 3d's than a full 4s a.In chromium, the electrons in the 3d and 4s orbitals rearrange so that there is one electron in each orbital. It would be convenient if the sequence was tidy - but it's not! Mn: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2 (back to being tidy again) Fe: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2: Co: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2: Ni:
Chromium is a chemical element with atomic number 24 which means there are 24 protons and 24 electrons in the atomic structure.The chemical symbol for Chromium is Cr. Electron Configuration and Oxidation States of Chromium. Electron configuration of Chromium is [Ar] 3d5 4s1. Possible oxidation states are +2,3,6. .
The chemical symbol of Chromium is ‘Cr’ Electronic configuration of d-block. In general, the outer electronic configuration of these elements is (n-1) d 1-10 n s 1-2. Here, (n–1) stands for the inner d orbitals which may have one to ten electrons, and the outermost ns orbital may have one or two electrons. Electronic configuration of CrChromium is an example of a period four transition metal with an irregular electronic configuration. The correct electronic configuration of chromium contains one valence electron in the 4s subshell and five valence electrons in the 3d subshell. Therefore, .
electron configuration of chromium The derivation concept for the electron configuration brings clarity to scholars. Chromium Number of Valence Electron. So, keeping the same in mind we here have the derivation concept of Chromium electron configuration. We know that Chromium has an atomic number of 24 so the first two electrons of Chromium will .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer .In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule . The electron configuration of the central chromium atom is described as 3d 6 with the six electrons filling the three lower-energy d orbitals between the ligands. The other two d orbitals are at higher energy due to .
Gaseous chromium has a ground-state electron configuration of 3d 5 4s 1. It is the first element in the periodic table whose configuration violates the Aufbau principle. Exceptions to the principle also occur later in the . Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Electron Configuration For CrThe electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .

The electron configuration of Chromium is 1s2 2s2 2p6 3s2 3p6 4s2 3d4. Chromium is the chemical element located in the periodic table of elements, it is located in group 6, its atomic number is 24 and it is represented by Cr. This metal is mainly used in metallurgy. Its name is derived from the Greek chroma which means color and refers to the . Some elements do not follow the Aufbau principle, there are some alternate ways that electrons can arrange themselves that give these elements better stability. Using the Aufbau principle, you would write the following electron configurations Cr = [Ar] 4s^2 3d^4 Cu = [Ar] 4s^2 3d^9 The actual electron configurations are: Cr = [Ar] 4s^1 3d^5 .
The ground state orbital diagram of chromium is:. Ground state electronic configuration chromium 3+ electron configuration. The electronic configuration of Cr 3+ is 1S 2 2S 2 2P 6 3S 2 3P 6 3d 3 4S 0.The number of electrons in the Cr atom is 24, and its outermost atomic orbitals are 4s 1 3d 5.So removed an electron from the 4 .
electron configuration of chromium|Electron Configuration For Cr
PH0 · What is the electron configuration of chromium?
PH1 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH2 · Electron Configuration for Chromium (Cr and Cr2+, Cr3+ ions)
PH3 · Electron Configuration For Cr
PH4 · Electron Configuration For Chromium
PH5 · Chromium Electron Configuration (Cr) with Orbital Diagram
PH6 · Chromium (Cr)
PH7 · Chromium
PH8 · 2.2: Electron Configurations
PH9 · 1.9: Electron Configurations for Transition Metal Elements